Abstract
Background: Lenalidomide (Len) has been approved for anemic patients with Del(5)q syndrome and induces high response rates. However, patients exhibiting Del(5)q abnormality, but not classified as Del(5)q syndrome or presenting additional abnormalities over Del(5)q are not usually treated with lenalidomide and the experience on these patient groups is limited.
Aim: We investigated response rates and duration of response to Len in 67 MDS patients, exhibiting Del(5)q, but not classified as Del(5)q syndrome and compared their clinical and laboratory features with those of 120 patients with Del(5)q syndrome, treated with Len the same time period. Data were collected from the Hellenic (Greek) National MDS Registry database.
Methods: Patients were classified in four groups: Group-1 (N=120), with the typical Del(5)q syndrome, Group-2 (N=25), exhibiting Del(5)q alone, but not fulfilling criteria for Del(5)q syndrome, Group-3 (N=22), with Del(5)q plus 1 additional cytogenetic abnormality and Group 4 (n=20), exhibiting Del(5)q plus >1 additional cytogenetic abnormalities. Results were expressed on an "intention to treat" basis. Patients were considered evaluable for response if they had completed at least 2 cycles of Len treatment.
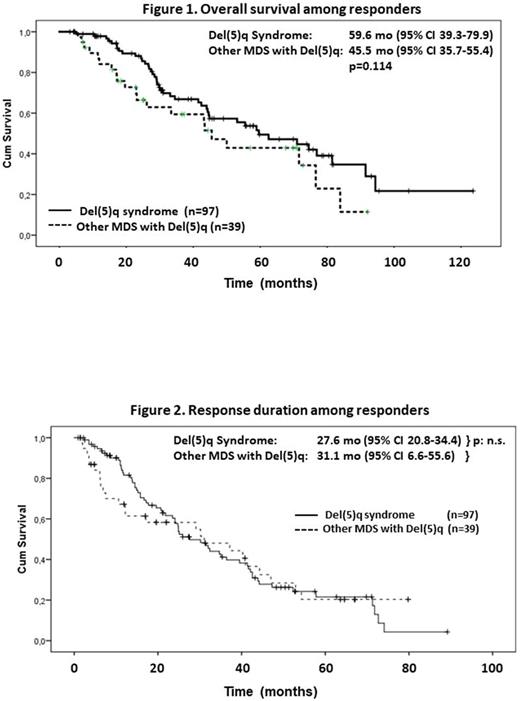
Results: Group-2 patients were significantly younger, compared to those of all the remaining Groups (p<0.05 for all comparisons) and the female to male ratio was higher in Group-1 (4.8 vs 2.1, 2.2 and 2.3 for Groups-2, -3 and -4, respectively). According to WHO classification patients of Group-2 were classified as RAEB-1 (n=16), RAEB-2 (n=6), CMML-D (n=1) and AML (n=2), patients of Group-3 as RA (n=7), RCMD (n=8), RAEB-1 (n=4), RAEB-2 (n=1) and AML (n=1) and patients of Group-4 as RCMD (n=5), RAEB-1 (n=5), RAEB-2 (n=8), CMML-P (n=1) and AML (n=1). Overall, 22 patients (Group-1: 11, Group-2: 1, Group-3: 5 and Group-4: 5) were not evaluable for response. Among 165 evaluable patients a complete hematological response was obtained by 83/109 (76.1%) patients from Group-1, 16/24 (66.7%) from Group-2, 9/17 (52.9%) from Group-3 and by 4/15 (26.7%) from Group-4. Partial response was achieved by 14, 4, 3 and 3 patients from each group, respectively, comprising overall response rates of 89%, 83.3%, 70.6% and 46.7%, respectively. Collectively, a favorable response was obtained by 26/38 (68.4%) patients with excess of blasts or AML in Groups-2 and -3 or by 32/41 (78%) patients from Groups -2 and -3. The median duration of response was 22.3 months in Group-1 and 17.3 months in Groups -2 and -3 (p: n.s.). Median duration of response was higher for patients without excess of blasts (21.6 vs 12.2 months, p<0.01). Complete cytogenetic response (CCyR) was confirmed in 28/56 patients from Group-1 and in 9/29 patients from the remaining Groups. CCyR was associated with higher MCV decrease and with reduction or disappearance of bone marrow biopsy-detected lymphoid nodules in 26/45 cases. Kaplan-Meier estimates for median overall survival following Len start was 67.6 (95% C.I. 43.4-91.8) months for Group-1, 43.7 (23.5-63.9) months for Group-2, 26.4 (22.1-30.6) months for Group-3 and 21.7 (12.7-30.7) months for Group-4 patients (p<0.001). In multiparametric analysis, factors associated with longer survival were achievement of complete hematological response (p<0.001), shorter interval from initial diagnosis to Len start (p<0.001), younger patient's age (p=0.01) and deeper MCV decrease at best response (p=0.05).
Conclusion: Len may be highly effective, not only in patients with Del(5)q syndrome, but also in other MDS patients, exhibiting Del(5)q alone, or associated with 1 additional cytogenetic abnormality, even when excess of blasts is detected and might be considered as a treatment option in these MDS groups.
Vassilakopoulos: Roche: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Celgene/GenesisPharma: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding. Katodritou: Janssen: Honoraria; Takeda: Honoraria, Research Funding; Amgen: Honoraria; Celgene: Honoraria, Research Funding. Pagoni: Gilead: Honoraria; Janssen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; BMS: Consultancy, Honoraria. Angelopoulou: Janssen: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Celgene/GenesisPharma: Consultancy, Honoraria, Research Funding. Labropoulou: Amgen: Honoraria; Celgene/GenesisPharma: Honoraria; Janssen: Honoraria. Terpos: Amgen: Honoraria, Other: SC member, Research Funding; Janssen: Honoraria, Research Funding; Genesis/Celgene: Honoraria, Other: DMC member, Research Funding; Takeda: Honoraria, Other: SC member; BMS: Honoraria; Abbvie: Honoraria; GSK: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal